Introduction
Electrocatalysts are critical to increase reaction rates and control
selectivity in many electrochemical fuel production and consumption reactions.
In the Jaramillo Group, we develop new electrocatalyst materials for processes
including hydrogen evolution and oxidation, oxygen evolution and reduction, and
carbon dioxide reduction. These reactions are necessary for electricity generation
in hydrogen fuel cells, H2 production through water electrolysis, and
CO2 conversion to create useful fuels and chemicals. Our approach is
to understand the catalyst properties that control activity, selectivity, and
stability by combining catalyst synthesis and electrochemical performance
testing with physical and chemical characterization as well as computational
and theoretical modeling performed by collaborators. Using these insights, we
design new electrocatalyst materials with improved performance.
Hydrogen Evolution Reaction
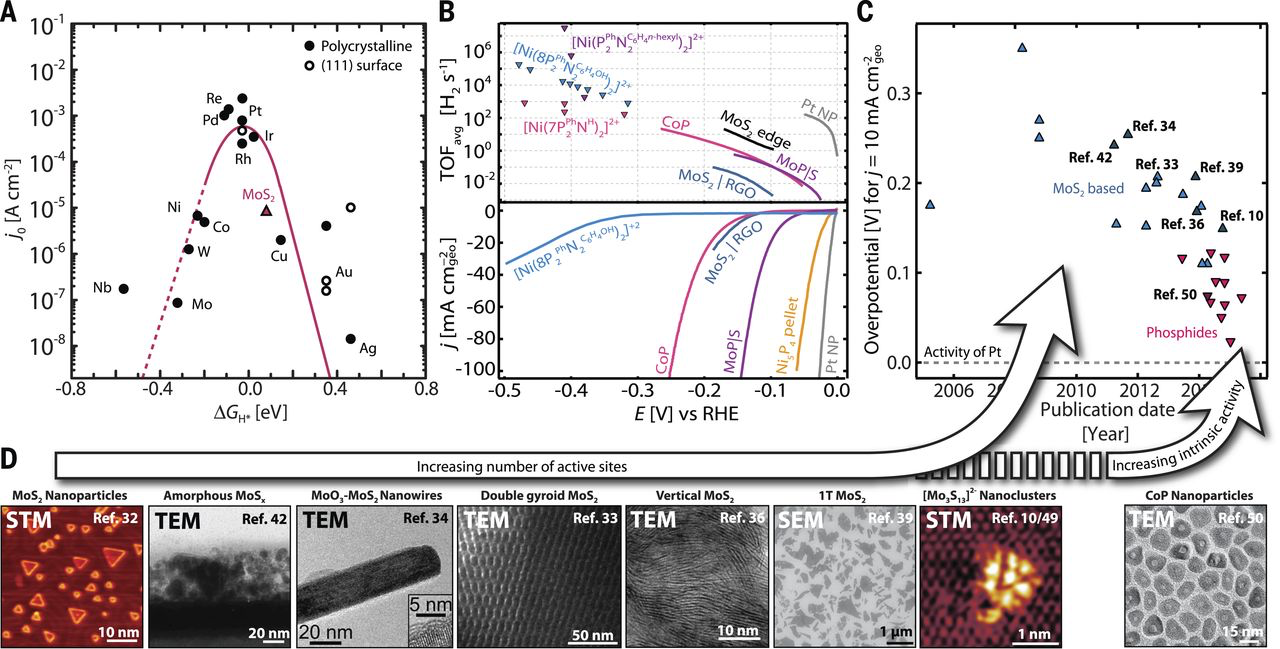
The hydrogen evolution reaction (HER, 2 H+ + 2 e− → H2) is the
cathodic reaction in electrochemical water splitting. The HER is a classic
example of a two-electron transfer reaction with one catalytic intermediate,
and offers the potential to produce H2, a critical chemical reagent and fuel.
Driving the HER with renewable sources of energy can lead to a sustainable
source of hydrogen fuel that can stored, transported and used in a zero-emission
fuel cell of combustion engine. Achieving high energetic efficiency for water
splitting requires the use of a catalyst to minimize the overpotential necessary
to drive the HER. Platinum is the best known catalyst for HER and requires very
small overpotentials even at high reaction rates in acidic solutions. However,
the scarcity and high cost of Pt limits its widespread technological use. We
study the fundamental material properties that determine catalytic activity
for the HER. By designing new catalyst materials for the HER, we have expanded
our understanding of the surface structures and properties that govern HER
activity and stability. By applying this knowledge towards next generation
catalyst design, we have developed several earth-abundant HER catalysts,
including sulfide- and phosphide-based materials, with activities approaching
that observed for platinum.
Oxygen Evolution Reaction
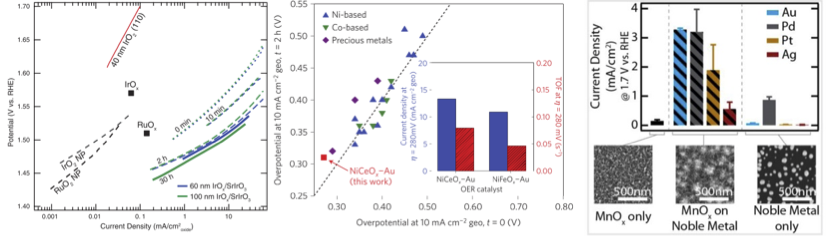
Image credits: L.C. Seitz et al., Science, 2016; Z.W Seh et al., Nature Energy, 2016; L.C. Seitz et al., J.Phys.Chem, 2015
The oxygen evolution reaction (OER, 2H2O → O2 + 4H+ + 4e-)
is the complementary anodic half reaction in electrochemical water splitting.
Requiring four proton and electron transfers per oxygen molecule, the OER is
the more complex of the two half reactions and is consequently responsible for
the majority of inefficiency in electrolyzer devices. In acidic media, only
Ir-based catalysts have shown high activity and stability for the OER. To reduce
dependence on expensive and rare precious metal catalysts, we aim to develop
higher performance, low Ir-content materials for the OER .
In alkaline media, catalyst material restrictions are relaxed and many first-row
transition metal oxides show high activity for the OER. We aim to developed high
performance OER catalysts , determine
to origin of enhanced activity through advanced characterization techniques ,
and incorporate high performance catalysts into devices .
The Jaramillo lab seeks to develop novel, high performance OER catalysts through
fundamental understanding of the structure-function relation of the catalyst surface
to enhance efficiency and promote increased commercialization of energy storage devices.
Oxygen Reduction Reaction
The oxygen reduction reaction (ORR) is a pertinent process in many electrochemical energy conversion and storage technologies, including fuel cells and rechargeable metal-air batteries. In acidic conditions, only platinum-based catalysts have shown sufficient activity and durability for practical purposes, although comes at a high cost and relies on limited global platinum supplies. Research in our laboratory focuses on designing improved activity ORR catalysts, with significantly reduced or eliminated platinum contents. Some strategies that have been employed to achieve this include the use of solid solution or intermetallic platinum alloys, shape controlled nanostructures to achieve preferential facet exposure, or the preparation of core-shell nanoparticles that can tune the interactions (i.e., binding energies) with ORR intermediate species. Our aim is to develop an improved understanding of the ORR process occurring on various surfaces, providing fundamental insight that can rationally guide our design of new catalyst materials.
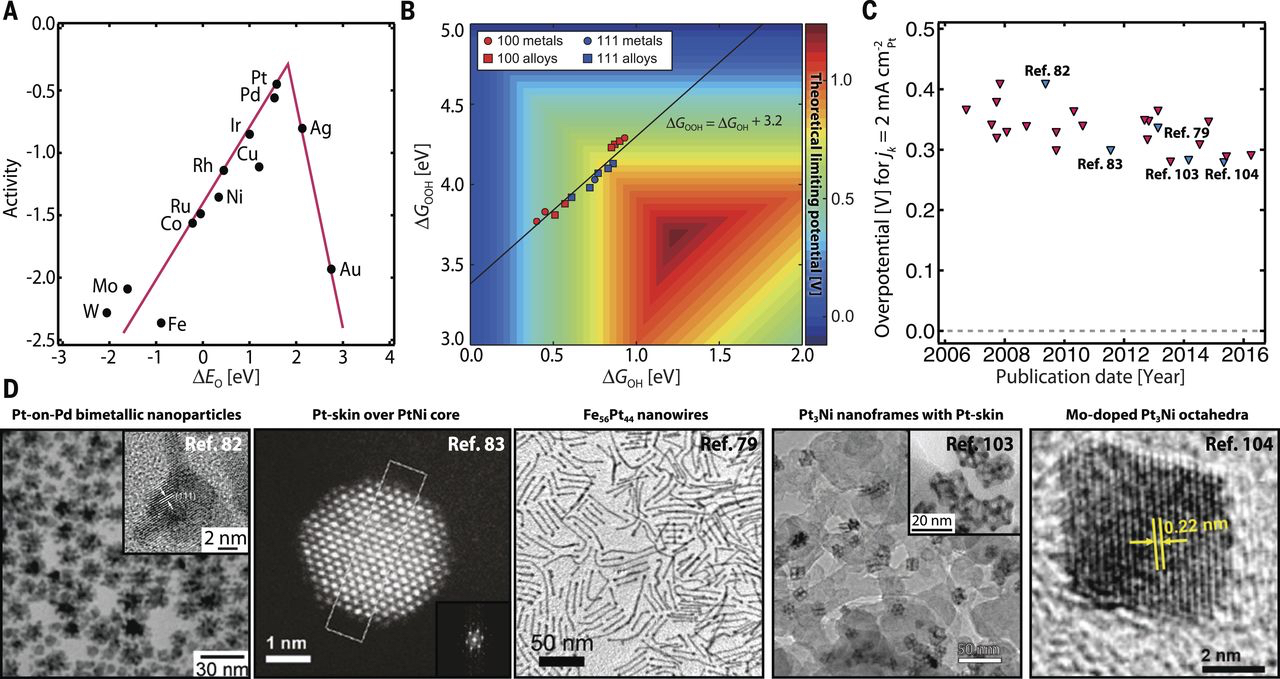
Image credit: Z.W. Seh et al., Science, 2017
In alkaline conditions, the ORR is a much more facile process and
provides opportunity for non-platinum catalyst alternatives, including metal
oxides, nanostructured carbons and silver-based catalysts. The ORR process on
these surfaces is poorly understood in comparison to platinum, and the activity
still lags behind that of platinum on a turn over frequency basis. We aim to
develop new material designs and develop a thorough understanding of the surface
structures and properties that influence intermediate adsorption energies and ORR
activity on various alkaline-based catalysts.
The ORR can also proceed by a 2 electron reduction mechanism, forming hydrogen
peroxide species. While this is undesirable from an energy efficiency standpoint
for fuel cells (only 2 electrons transferred per oxygen molecule to form hydrogen
peroxide as opposed to 4 to form water), this electrochemical reaction is advantageous
because it could replace the energy intensive anthraquinone process conventionally
used to synthesize hydrogen peroxide. This electrochemical synthesis could, for
example, be coupled to renewable sources of energy (i.e., wind or solar) to
provide on site generation of hydrogen peroxide. As a powerful oxidizer,
distributed production of hydrogen peroxide could play an important role for
drinking water treatment or sterilization applications.
CO2 Reduction Reaction
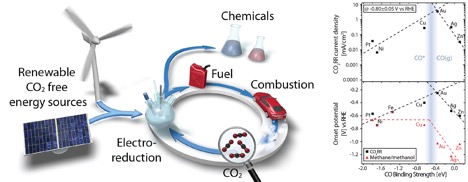
Electrochemical reduction of CO2 has a potential of
becoming a major contributor to sustainable production of fuels and chemicals
through the use of renewable CO2 free energy sources. However, the
development of an effective catalyst is vital, as there are currently no industrial
scale operations that utilize this technology due to the low energetic efficiency.
In our group, we focus on gaining fundamental understanding of the surface chemistry
by tuning some of the key catalyst characteristics, such as the composition, the
surface structure, and the morphology, as well as other factors, such as electrolyte
composition and reaction conditions. By implementing the insights gained from our
work, we aim to design effective catalysts that would allow for the industrialization
of the technology.
